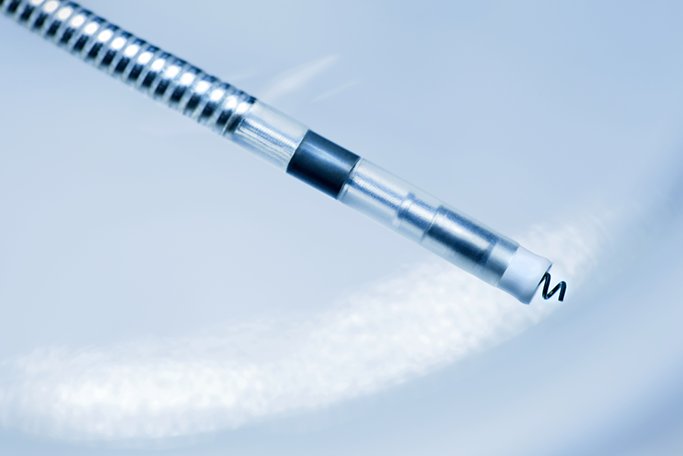
of the two trials, a total of 3,933 LINOX ICD leads have been implanted in 3,840 patients at 146 US study centers.
"The results of this large scale, prospective analysis should provide assurance to both implanting physicians and their patients that the magnitude of scientific evidence shows these leads to be safe and reliable," commented lead author Dr. Eric Good, formerly with University of Michigan Cardiology, US. "Their 'real-world' performance and survival rates are similar to other well-performing leads reported in the literature."
The ongoing observational GALAXY and CELESTIAL registries collect lead safety and reliability data for five years post-implantation, with all protocol-defined procedural and system-related adverse events recorded at each follow-up. The estimated cumulative survival probability for Linox leads at five years is 96.3 percent, while for Linoxsmart leads at four years, it is 96.6 percent, both well within industry standards for safety and reliability. Long-term data on the Linox and Linoxsmart ICD leads from the ongoing GALAXY and CELESTIAL registries is now available from the Journal of Cardiovascular Electrophysiology.
“The scale and thoroughness of the GALAXY and CELESTIAL registries demonstrate BIOTRONIK’s deep commitment to evaluating our ICD leads’ long-term durability, a product of an intensive design process followed by extremely rigorous testing,” commented Manuel Ortega, Senior Vice President at BIOTRONIK. “As good is never enough for us, we constantly strive to further refine our products’ reliability, performance, and usability. Our philosophy has always been incremental improvement with a strong focus on durability and performance. That’s what our engineers in Germany, Switzerland, and the US are constantly working hard to achieve.”
Clinical Trials Registration Information: ClinicalTrials.gov NCT00836589 and NCT00810264
For more information, visit: www.biotronik.com
* Investigational device: Limited by United States law to investigational use.