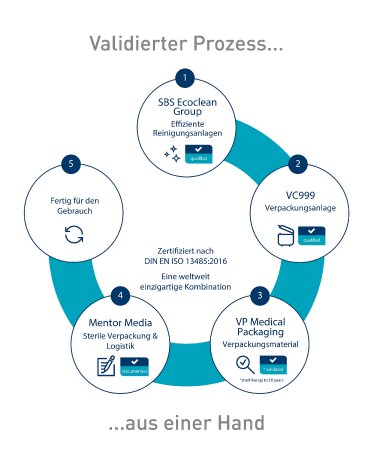
The SBS Ecoclean Group and VC999 Medical have concluded a worldwide sales and service cooperation with the aim of minimizing both the time and financial effort involved in certifying and validating the cleaning and sterile packaging of medical technology products. Ecoclean supplements its turnkey range for medical technology with the MDR and GMP-compliant packaging systems of VC999 Medical, which, depending on the application, already contain pre-validated packaging materials (PAPE, Tyvek and paper/foil). "The packaging machines and materials from VC999 Medical have been established on the global market for decades and ideally complete our portfolio," reports Fabio Cordaro, Global Business Development CLP and expert for parts cleaning in medical technology at Ecoclean. "This enables us to plan and supply manufacturers of medical technology products, such as implants and instruments, with complete turnkey solutions, from preliminary and intermediate cleaning during production to final cleaning and transfer to the clean room to sterile packaging."VC999 Medical is a business unit of the Swiss VC999 Verpackungssysteme AG that specializes in packaging and is represented in over 70 countries around the world. The family-run company is the only manufacturer of packaging solutions in the world to have its own packaging validation with a shelf life of ten years. “On the one hand, the fact that it is also a global player in the medical technology market spoke in favor of the cooperation with Ecoclean. On the other hand, we got a partner on board who covers the process immediately before packaging," explains Jürgen Klein, who is responsible for product management at VC999 Medical. "This enables us to offer customers quick and effective cleaning and packaging of their products, which contributes to a more efficient production." The added value that results for customers from the close cooperation includes, on the one hand, increased safety for the processes of professional cleaning and sterile packaging, taking current legislation into account. On the other hand, the effort for certifications and validations is minimized so that they can be used quickly. Added to this is the simple adaptation of the solutions for production sites in different countries and across continents.For optimal process design and coordination, the partners have mapped a complete process chain from preliminary, intermediate and final cleaning, including clean room and packaging at Ecoclean's Center of Competence in Monschau (Germany). Corresponding trials with original products can be carried out and analyzed in laboratories.The close cooperation between Ecoclean and VC999 Medical is also underpinned by joint trade fair appearances.
www.ecoclean-group.net, www.vc999medical.com