The Patient Safety Day aims at increasing public awareness. It is organized by the German “Aktionsbündnis Patientensicherheit” and supported by the German Federal Ministry of Health. Focus this year is on “medication safety”. Hospitals, nursing homes and other health organizations are invited to participate as well as industries, manufacturers and consultants.
FOBA Laser Marking + Engraving, leading manufacturer of laser marking machines, is part of “MedicalMountains”, a cluster initiative with over 200 member companies, which represents quality and innovation in medical technology. FOBAs high-performing laser marking machines form an important part of the production lines of medical device manufacturers worldwide.
“Patients’ safety is every medical company’s final concern”, Yvonne Glienke, Chairwoman of “MedicalMountains”, explains. “For that reason we offer trainings and information seminars on related subjects like CE marking, biocompatibility, technical documentation and the European medical device regulation”, Glienke continues. “All implemented measures’ target is to ensure that patients benefit from safe products and services.”
The latest changes in the European medical device regulations will be subject of a training offered by “MedicalMountains” in the first quarter of 2017, providing help and information for management to adapt necessary changes in their businesses.
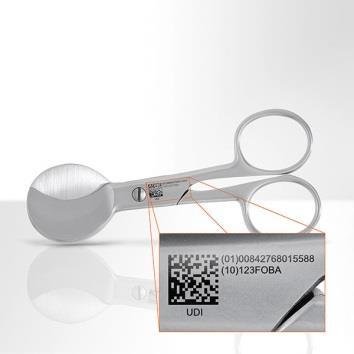
Lately extended official directives like UDI (Unique Device Identification) regulations by the FDA (U.S. Food and Drug Association) or the European Medical Device Regulation MDR are intended to better control the big variety of products on the medical market, to store information in a central data base and protect quality standards. Not only products of low risk like dressing material or stethoscopes are affected, but also middle to high risk devices like hearing aids, medical tubes, intravenous catheters, scalpels or implants.
Direct part marking (DPM) on medical devices ensures continuous traceability from manufacturer to patient and is mandatory for many industries. In this context, “MedicalMountains” provides support for its member companies to find adequate marking technologies.
Laser marking appears to be the most reliable technique for medical devices, which require biocompatible and hygienic marks. High marking quality with excellent long term resistance ensures traceability throughout the whole product life cycle. In terms of medical implants for example, these marks contain data on time and place of production, further processing, attending physicians, time and place of surgery and patient data. All information can be taken directly from the product and transferred further into hospital and other documentation systems.
Manufacturers benefit from FOBA’s HELP (Holistic Enhanced Laser Process), a vision-based marking system that verifies parts and marking contents before, during and after marking. An efficient and nearly error-free production can thus be guaranteed and production costs can be reduced by up to 80 percent.
Laser marking is most suitable for direct part marking on materials most medical devices are made of, like titanium and other metals or different plastics. Also highly sensitive silicone products can be adequately marked by FOBA UV-lasers or the pulsed Ytterbium fiber-lasers from the FOBA Y-Series.
The flexible vision-based FOBA marking systems solve the current demands of medical device manufacturers for industrial parts marking.
• Stable marking processes and constant high marking quality enable increasing productivity
• Flexible use for small series and changing products
• Quick integration into production lines and manufacturing systems
• Permanent and reliably legible laser marks and code re-verification support safe product traceability.
Find further information about application examples from medical industries on the FOBA website: http://www.fobalaser.com/...
Find further information on MedicalMountains on http://www.medicalmountains.de