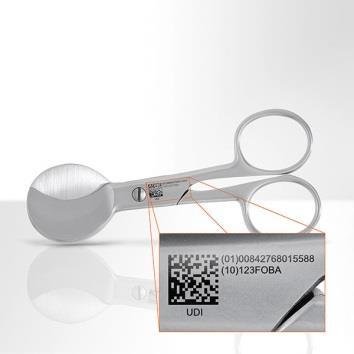
Manufacturers of medical devices face multiple challenges. There is on the one hand the Unique Device Identifier (UDI), mandated by the FDA. The Administration requests a machine and human readable code on more and more medical devices that has to be marked on the product. On the other hand there are a rising number of varying parts that need to be marked and cutbacks in health budgets. FOBA, aware of those challenges, developed therefore flexible, cost-efficient and reliable laser systems.
At the MD&M East FOBA is showcasing its high precision and cost-effective laser marking workstations FOBA M2000 with integrated IMP vision (Intelligent Mark Positioning). It is an integrated high-speed camera system which automatically detects devices and their positioning, and aligns the mark relative to the part’s position. With IMP manufacturers achieve a zero-defect marking quality which could grant them a decisive advantage in a market as competitive as the medical sector.
FOBA’s vision solutions are part of the closed-loop laser marking process HELP (Holistic and Enhanced Laser Process) which covers pre- and post-mark verification. HELP is a process of three stages. First stage is the part validation, the pre-mark verification and the mark alignment. After the second stage, the laser marking, comes the third stage with mark verification, optical character verification OCV and the 2D code validation including UDI. At MD&M East, FOBA will show the HELP process with the M2000. In addition to that, the company will also showcase a M1000, a smaller stand-alone-system for flexible and reliable laser marking.
Register for free at http://page.fobalaser.com/...