This post will look at:
- continuous bioprocessing modes
- cell retention methods
- how to use the antifoam pump for harvesting
- configuring feed and harvest pumps
- making aseptic connections
The main aim of continuous cultivation is to maintain a continuous flow between the addition of fresh media and the removal of cell suspension from the bioreactor simultaneously in order to maintain a constant reactor volume (Ozturk, 2006). In this way fresh nutrients are added, the product extracted, and possibly toxic by-products removed. All culture parameters remain constant, which demands additional control. It is also important to consider the danger of washing out the cells, which needs to decide for an appropriate dilution rate versus growth rate ratio.
Three types of continuous culture are described below:
Chemostat
Cell growth is limited and controlled by a nutrient addition (glucose, oxygen, glutamine and others) and depleted media with growth inhibitors is removed at approximately the same rate.
Turbidostat
A turbidostat dynamically adjusts the flow rate (and therefore the dilution rate) to keep the turbidity inside the vessel constant. The turbidity caused by cells in the culture medium can be tracked with sensors that measure light backscatter. Keeping the turbidity constant presents a new challenge for continuous operation. because cells need to be periodically removed independent of adding fresh medium separate from the harvesting process. Unlike chemostat operation, the cells need to be kept in the vessel for much of the process. When the culture density is too high, they must be removed, all while keeping a constant working volume.
Perfusion
This type of continuous bioprocessing mode is based on either retaining the cells in the bioreactor or recycling the cells back to the bioreactor. Here, fresh medium is provided and cell-free supernatant removed at the same rate (Ozturk, 2006). Various cell retention methods exist. Furthermore, in this mode the cell density increases constantly depending on the chosen media perfusion rate, also known as dilution rate factor D. The cell growth is limited to the nutrient or oxygen constraints or by waste product inhibition, leading to a quasi-steady-state of cells, metabolites and product concentration (Ozturk, 2006).
2. Cell retention methods
Few cell retention methods are available. Here the most frequent ones are discussed:
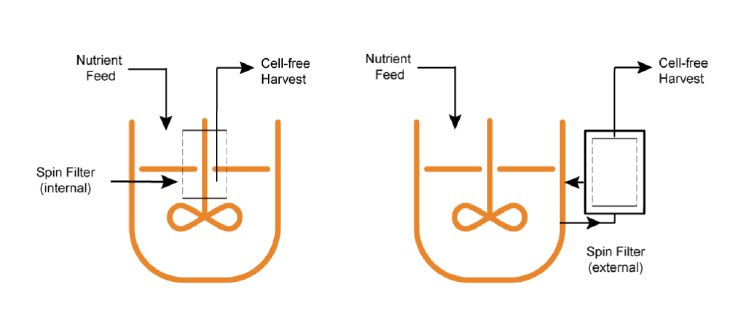
Spin filter
This kind of cylindrical filter can be either attached internally to the drive shaft or externally to the bioreactor. The mesh size is small enough that the cells cannot pass through it to the inside. Depleted cell-free medium is removed by the dip tube inside the spin filter and fresh feed is added to balance the removal.
Alternating Tangential Flow (ATF)
The principle of ATF is based on alternately pumping the cell suspension to the filtration system and then back to the bioreactor by using a fast diaphragm pump. For the separation of cells and product a standard hollow fiber membrane is applied [1].
Tangential Flow Filtration (TFF)
TFF is also known as cross-flow filtration, where the suspension is passed parallel to the filter system. Due to a pressure difference the filtered particles that are smaller than the filter pores pass through, whereas the larger components are retained and recycled back to the vessel.
Both versions of Tangential Flow Filtration are incredibly important because the technologies that they rely upon are low-shear and they do not damage the cells the way conventional peristaltic pumps may.
3. Setting up the antifoam pump for harvesting
Most bioreactors come with built-in peristaltic pumps, usually with one linked to antifoam. The antifoam pump typically adds liquid to the vessel from a reagent bottle and this happens when the foam probe is activated. In continuous bioprocesses, culture volume needs to be removed from the vessel into a specific harvest bottle. The easiest way to set this up is simply reverse the connections to the antifoam pump.
Once the vessel is autoclaved, the antifoam probe body should only ever be pulled upwards to support aseptic operation and the height of the probe should be adjusted with maximum stirring and airflow set. Both agitation and gas flow can raise the height of the column of liquid in the vessel.
4. Configuring the feed pump
Feeding can only start when the culture has grown to a certain density, which means that a batch phase of operation must be planned. Once this is over, feeding can be started manually or by timed events controlled by software. The feed rate from the media bottle into the vessel can be set manually or remotely via a script within the SCADA (Supervisory Control And Data Acquisition) software.
The simple rules for feeding are:
- Most continuous cultures run as chemostats. Addition of only one substance limits growth in the culture. The maximum feed rate will depend on the growth of the specific microbe on a defined growth-limiting substrate. It is important to take care that the cells are not washed out, which means the liquid exchange should be slower.
- The media exchange is defined by the dilution rate (D), usually in units per hour (h−1). It describes the relationship between the flow of medium into the bioreactor (F), that can be expressed in L·h−1, and culture volume within the bioreactor (V) in L
- To reach a culture at steady state, the Dilution rate (D) must be equal the specific growth (µ) thereby keeping a constant biomass concentration inside the bioreactor.
The antifoam pump is now a harvest pump and needs to be set up as such:
- The take-off (harvest) must be set to a faster rate than the inlet feed. This makes sure the vessel cannot overfill.
- As the antifoam pump works on a shot and delay basis, this usually means running a longer ON time and a shorter OFF time than usual (dose and delay).
- The direction of flow is from a dip tube in the vessel through the antifoam pump and into a harvest bottle.
6. Making aseptic connections
As cultures can last for weeks or months, there is no way to only use a single feed and harvest bottle. If they were large enough, they would be too heavy to move easily, once filled. So, exchange of feed and harvest bottles is necessary.
Luer connectors make this task secure and rapid. The new bottle is fitted with one part of a Luer connector, filled and autoclaved. The open end of the connector can be covered with aluminium foil to reduce the risk of contamination. When exchange is needed, the existing Luer connector is sprayed with alcohol and opened. The new connector is uncovered and rapidly twisted onto the existing part. With a little care this operation can be securely repeated many times for microbial processes. For cell culture applications working with a tube welder ensures a sterile exchange of the bottles. The tubing can then be quickly primed.
Summary:
The theory of continuous culture is well understood. The practical details to make a success of it are not so easy to find. Some of the key elements have been mentioned to help you i.e.
- How to simply change the use of an antifoam pump to make it run as a harvest pump.
- Configuration of the feed and harvest elements of a continuous process.
- Dealing with the need for many aseptic connections during the lifetime of the process.
- Condensing the key information into a simple workflow for a chemostat.
This article has been published on the INFORS HT blog.
References:
Ozturk S. et al., Cell culture technology for pharmaceutical and cell-based therapies, (2006)