“The FDA approval of mediCAD® 4.0 and its 3D products for hip and spine marks an important step in bringing new features to our software currently available in the U.S.”, said Jörn Seel, CEO of mediCAD Hectec GmbH, Germany. “The interest in these new versions of our software has already been very high in other markets around world and has expanded the capabilities of preoperative, digitized planning for surgeons in the orthopedic field. With this approval by the FDA, we now are pleased to bring these exciting new product developments to the U.S. market and look forward to continuing to work with orthopedic surgeons to solve their planning needs.” The mediCAD® portfolio of products are software tools that allow orthopedic surgeons to efficiently and safely plan joint, trauma and spine operations in a fraction of the time required for conventional planning. Further, with the automatic archiving of critical information and complete traceability of findings, mediCAD® provides a framework for monitoring and documenting surgical procedures.
In this latest version of mediCAD® approved for use in the U.S., there are several new key features added to simplify the work including automatic repositioning in the hip planning; automatic recognition of the femur axis when measuring the offset; and a favorites list in the implant database. Additionally, the knee module now offers the possibility to place templates in both AP and ML views simultaneously.
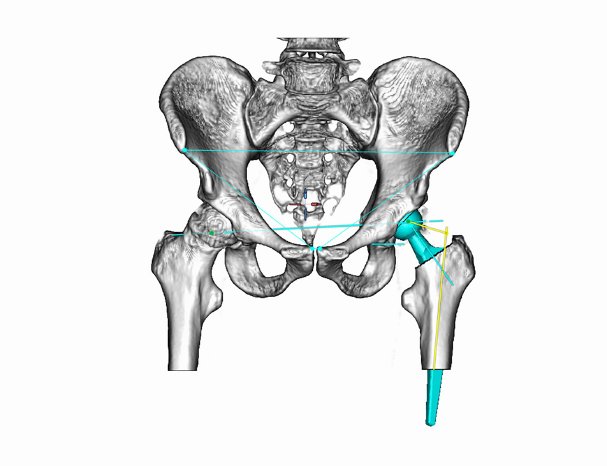
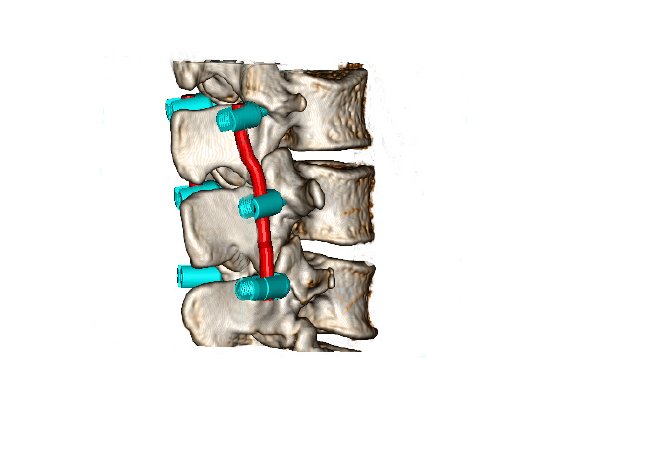
Brand new to the U.S. market is mediCAD® Hip 3D and Spine 3D. These software modules allow surgeons to plan in a 3D environment utilizing CT scans, which are most useful in analyzing and planning complex cases.
With the mediCAD® solution and the approval of these products for use in the U.S., the company continues to set innovative milestones worldwide in supporting surgical orthopedics and maintains its position as a global leader in the field of medical software solutions.