Innovative and tried and tested system solution
With more than 500 installations at leading pharmaceutical and medical technology companies, weisstechnik® is an experienced system partner for clean room technology, stability testing and monitoring. All services are provided from a single source, ranging from the planning and implementation to qualification and service. The S!MPATI® monitor monitoring system has been specially developed for the pharmaceutical industry, pharmacies and biotechnology companies, and can also be used in medical technology and temperature-controlled logistics (GDP). With the additional TimeLabs® module, the system allows image-based, non-GMP-compliant recording of processes that can be linked to the visualisation with measurement data parallel to the standardised documentation of measured data.
Significantly more than monitoring and recording
The core feature provided by S!MPATI® is its secure monitoring and documentation of process parameters and production conditions. In addition, the flexible control and documentation software can also be used for existing devices, enabling uniform monitoring.
Extensive basic features
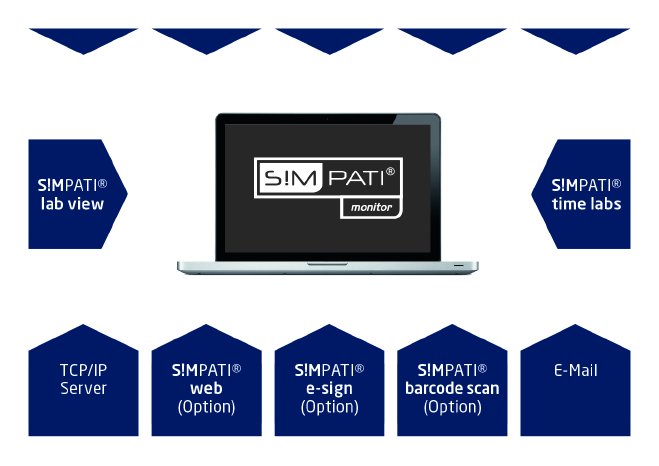
Via its connected measuring technology, even the basis version of S!MPATI® monitor measures parameters such as room/differential pressure, temperature and relative humidity and evaluates these quickly and precisely. In addition, air speed, airborne particle concentration, door contacts/digital inputs and standard signals can also be monitored and documented. The results are clearly displayed on the user-friendly user interface.
Options: Visual documentation, batch tracking and more
For visual documentation, S!MPATI® can be optionally extended with TimeLabs®. For seamless batch tracking, electronic signing is available, for example, via the eSign module and necessary batch tracking via the barcode functionalities. In addition, the S!MPATI® range offers numerous other automatically and seamlessly networkable components. Different systems can be integrated using a uniform user interface.
Framework conditions and qualifications
S!MPATI® ensures the reliable and legally compliant recording of data according to GMP, GXP, GDP, GLP and GCP in compliance with the FDA 21 CFR Part 11 and EU GMP Annex 11 specifications in accordance with the manufacturer's declaration. In addition, S!MPATI® helps to meet diverse requirements for monitoring processes in specialist system construction. The GMP-compliant system qualification includes risk analysis (RA), design qualification (DQ), installation qualification (IQ) and operational qualification (OQ), whereby the documentation can be tailored according to customer-specific standards.
Find out more!
Visit us at
Medtec Europe from 4 to 6 April 2017 in Stuttgart
Hall 3, E30
Schunk Group
The Schunk Group is an internationally active technology company with around 8,100 employees in 29 countries. The group offers a wide range of products and services in the areas of carbon technology and ceramics, environmental simulation and air-conditioning technology, sinter metals and ultrasonic welding. In 2015, the Schunk Group achieved a turnover of 1.065 billion euros, thus breaking the billion barrier for the first time.