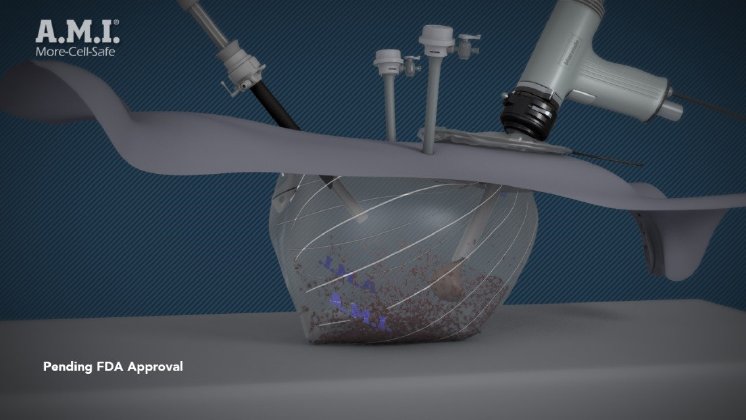
A.M.I.’s containment system will provide a layer of safety for surgeons and patients benefiting from the use of the Blue Endo power morcellator. A.M.I. developed a containment system for power morcellation which has been sold in European and some non-European markets since 2015 when it received the CE Mark. Published evidence for the More-Cell-Safe containment system asserts that it significantly reduces cell spread (based on procedures with an animal model). Following this initial test series, it was introduced to key European markets and continues to be used successfully on patients. More-Cell-Safe is not yet FDA approved, but will be submitted shortly for clearance in the US.
The More-Cell-Safe system consists of a dual port bag. One of the ports is for the morcellator, the other is used for the optics to view the procedure. This second port has added protection by including a sleeve (VisiShield) to cover the optics and a special closing of the second port that was developed with the request to eliminate cell spread. The system, in non-US locations, is encouraging surgeons to choose safer laparoscopic surgery over a laparotomy. Uteri and myoma of sizes more than 1 kg have been morcellated using the system.
Blue Endo has chosen A.M.I. because of the superiority of More-Cell-Safe compared to other solutions for containment. Both Blue Endo and A.M.I. hope that the partnership that combines two superior products will pave the way back to state-of-the-art laparoscopic surgery and use of power morcellation. Ted Sullivan, president of Blue Endo states that “we want our surgeons to use the safest and most effective devices for morcellation. We are impressed with A.M.I.’s product and look forward to offering it to our customers in the US.”
Marc Jablonowski, managing director of A.M.I. (headquarters in Austria), states that “Blue Endo is an ideal US partner for A.M.I. because of its superior product that has proven to work smoothly with A.M.I.’s More-Cell-Safe in Europe. We are very excited to be working with Blue Endo to provide a safer solution to laparoscopic power morcellation.” Andrew Bendheim, managing director of A.M.I. Inc, Boston, adds that “Blue Endo’s market knowledge and distribution network together with their strong focus on customer education forms the ideal basis to introduce such an innovative procedure to US customers.”
Blue Endo, headquartered in Lenexa, Kansas, specializes in the development and marketing of leading edge minimally invasive surgical products and services. Blue Endo’s MOREsolution tissue morcellator is one of many products offered for gynecologists.