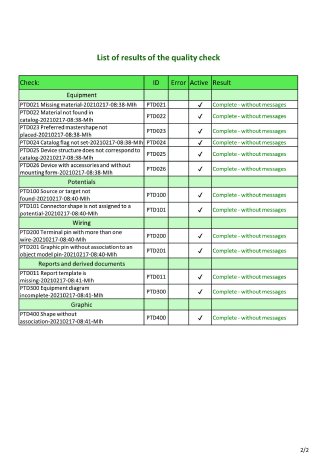
Signed and sealed quality
Engineering experts regularly check whether everything is correct during the design process. Supplied documentation is also checked. The visual inspection, which is still common even during final comprehensive tests, involves an enormous amount of time – without any guarantee of complete accuracy. EB, however, cannot "overlook" anything. Thanks to object orientation and a universal data model, all disciplines involved are combined in the system. It knows all the logic and links. The new quality management not only checks automatically for inconsistencies or non-compliance with requirements, it also automatically creates a list of all discrepancies per check and describes them. You can navigate to the conspicuous object directly from the list and correct the error. If everything is correct, the delivered project contains a certificate in the form of a test sheet with a green seal, which proves its correctness and is visible at first glance. Instead of weeks of work, the check takes a few hours at most. While initially developed for energy technology plants, this new QA framework can now also be used in other industries.
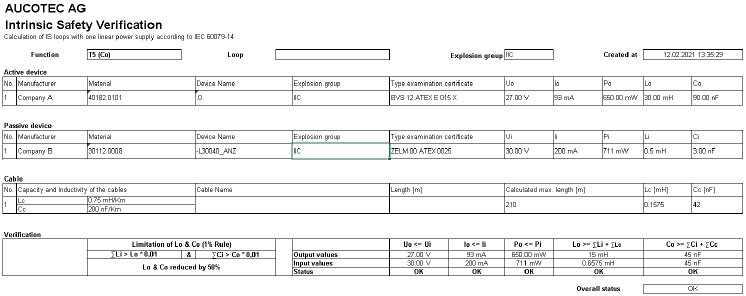
Safe Ex-i calculation by clicking
Where explosive atmospheres can arise, electrical sensors and actuators and their circuits must not cause ignitable sparks or excessively high surface temperatures. The intrinsic safety (Ex-i) of all associated equipment, lines and circuits must be reliably proven for the operating license, which is a major responsibility!
In EB, Ex-i data can be directly catalogued and maintained in order to design intrinsically safe circuits. In addition, intrinsic safety can be calculated and documented directly in the system by an Assistant, also as a typical. Up to now, this has usually happened in an extra tool in which external catalogue data has been entered. The Ex-i-Assistant, however, calculates all relevant circuits of a plant in accordance with IEC 60079-14 at the touch of a button, in only one process if required. EB keeps all information together, from the device and prototype test certificates via loop diagrams up to the calculation documentation. This saves laborious searching and error-prone data transfers.
Electronically signed and certified - FDA compliant
Absolutely flawless and legally-compliant documentation is required for plants in various industries. EB supports this requirement with multiple capabilities that meet even the strict regulations of the U.S. Food and Drug Administration (FDA), which has set standards worldwide. (Title 21 eCFR Part 11) The required e-recording covers EB with cross-disciplinary tracking of all changes to each asset, including its complete change history. Due to its multi-layer architecture, this information can also be accessed in a securely encrypted manner via a web service. In addition, EB now also enables legally-compliant e-signatures on plant documents. This includes a link to the certificate of signature authorization, time stamps, identification codes and password-protected accesses, among other things.
Aucotec at virtual Hanover Fair ‘21: Access data as of beginning of March via www.aucotec.com