The Horizon Impact award celebrates outstanding EU funded projects that have used their results to provide value for society across Europe and beyond. The MACH project was funded by the European Institute of Innovation & Technology EIT Health programme, and led by Professor Carina Benstöm from the RWTH Aachen. “This program successfully brought together experts from academia, healthcare and industry to work towards a common goal – to improve the lives of children waiting for a heart transplant” explains Professor Benstöm.
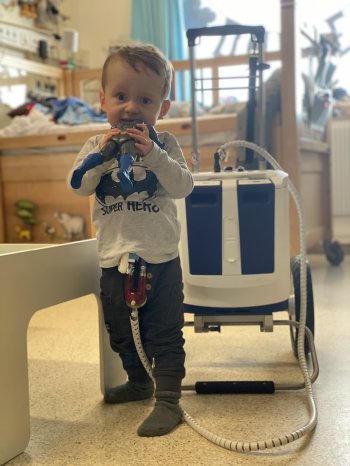
The EXCOR Active driving unit provides mobility to children with end-stage heart failure waiting for a heart transplant. Children can spend months and sometimes several years in hospital waiting for an organ. The goal of this project was to reduce the stress and impact of a long term hospitalisation on the children and their families, by offering them mobility and autonomy in their daily lives in the hospital environment. Children who have been very sick need to recover, build up muscles, explore and play. This is now possible with the new mobile device.
During the waiting time for a donor heart, these young heart patients are looked after by a team of dedicated nurses, intensive care doctors, surgeons, cardiologists, occupational therapists, physiotherapists, play leaders and psychologists who help the family to cope and to become experts in their child’s care. “Patients and families were given a voice in the project through interviews discussing how to make their experience more positive. The device and the new learning platform will benefit the family as well as the child waiting for a donor heart” says Dr Emma Simpson from Newcastle’s Freeman Hospital, whose team worked with researchers from Newcastle University.
The Berlin Heart EXCOR® Pediatric is the only Ventricular Assist Device approved worldwide for short to long term support in children of all ages with heart failure*. This project supported development of a smaller, lighter more mobile driving unit with a longer battery time by switching from a compressor technology to piston drivers, which are lighter and quieter. Since attaining CE Mark last year, the “EXCOR Active” has supported the first patients in Germany. “At Berlin Heart, we understand that patient-centred innovation requires close collaboration between engineering, health care professionals and end-users” states Sven-Rene Friedel. “We are happy to see the positive impact of the EXCOR Active recognized by such a prestigious award” adds Dr Ares K. Menon, Managing Director of Berlin Heart.
The 10.000€ prize money from the award will be donated to projects such as a facility that supports families staying near their hospitalised children in Munich and key initiatives with long-term benefit to patients at Freeman Hospital, Newcastle.”
For more information regarding the award please see:
https://ec.europa.eu/info/news/commission-announces-winners-horizon-impact-award-2020-2020-sep-23_en
https://www.youtube.com/watch?v=xM6oQCd0LVE
*The access to some or all shown products may be restricted by country-specific regulatory approvals. The use of EXCOR® VAD for adults, RVAD-support, Excor mobile and EXCOR® Active is not FDA-approved.”