Calibration
The analytics of BioTeSys GmbH is accredited by the German Accreditation System (DAkkS) according to the international norm DIN EN ISO/IEC 17025. Consequently, all laboratory actions and processes follow a prescribed quality assurance system. Central requirements of the norm are i.e. a functional quality management system, skilled personal, traceability of all processes and validated analysis methods.
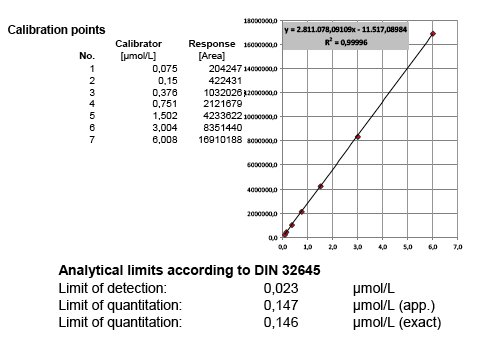
Method validation is the formal and documented proof that an analytical method is suitable for its intended use and meets the required specifications. It’s an important tool in the quality assurance and is demanded in the context of accreditation and admittance methods. The characteristics range, linearity, specifity, robustness, precision, recovery, limits of detection and quantification of the method are determined. Whenever possible, equidistant multipoint calibrating functions are tested according to DIN 32645. Aside a check for normal distribution, freeness of trends, comparability of variances and elimination of outliers, the adequate calibration function is calculated and LOD and LOQ calculated.
Accuracy, trueness and precision
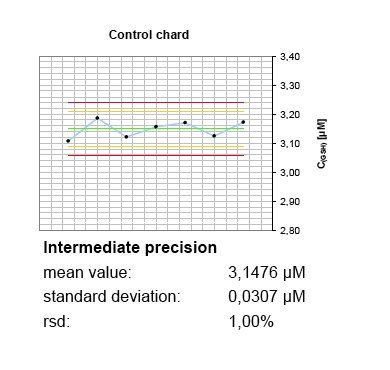
In the absence of certified reference materials, BioTeSys uses different charges and, if available, suppliers of calibration standards and control substances. Further, purity of standard materials is regulary checked to be in accordance with suppliers certificates of analysis with additional methods, favorable by UV/Vis-spectrophotometry. The whole system is calibrated and controlled before each analysis batch run and controls recorded in at least two chards covering different concentrations of the calibration range. Even small deviances of control values or calibration response can be identified before degradation of standards or deterioration of hardware is resulting in modified method performance or shifts of results over time.
DNA Test
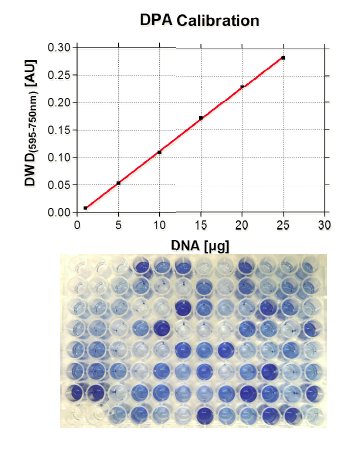
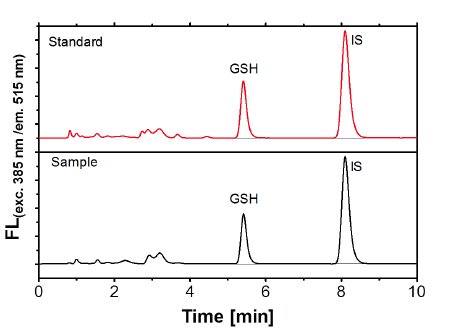
The quantification of parameters in cells is in general more difficult than in blood or serum, not only because of the challenge to measure analytes in the same amount of sample. To overcome this problem, BioTeSys uses a method to determine the amount of DNA in a given sample as a reference value to estimate sample size or the number of cells in it. This so called DPA assay, is based on the carbohydrate liberation from purine bases of DNA material by acidic hydrolysis. After incubation with diphenylamine, a blue colored complex is formed, which is specific for DNA and can be quantified by photometry. A special feature of this method is that the DNA does not have to be in an intact or native state, hence the DPA assay can be performed with the cell residue resulting from vitamin extractions.
Traceability and quality assurance
All steps of the analysis process are well documented and take place under close supervision. This starts with the arrival of the samples at BioTeSys, where all samples are registered and compared to the sample list on dry ice before being frozen at -80 °C. The storage temperature of the freezer is monitored and online systems control temperature, system status and reporting chains in the case of any alarm condition 24 hours a day. The freezer is, like all machines at BioTeSys, maintained and the performance checked regularly within a service contract. For the result quality important machines are available in a redundant spare setup to allow the lock of an analysis system without total loss of actionability and sample integrity. All documents of the quality management system like written resources, standard operation manuals, lists, process descriptions and so on are online available throughout all working stations and thus guarantees unitized handling. Personal is permanent under continuing education and working procedures are optimized through internal and external audits.