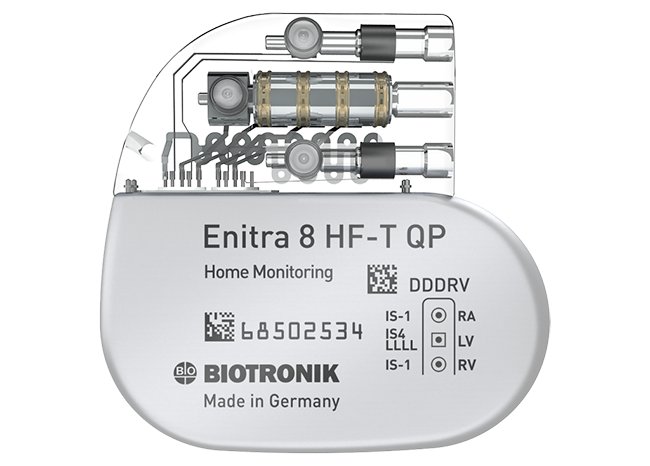
The patient, a woman with a history of heart failure and complete heart block, had an Enitra 8 HF-T QP device implanted on July 27 at Great Western Hospital in Swindon, United Kingdom. The operation was a complete success and she is recovering well. “The anatomy of this patient and the venous access presented a particular challenge, so the small size of Enitra and the quadripolar left ventricular lead were paramount in the success of the procedure,” explained Dr. Paul Foley, Consultant Cardiologist. “I was also personally very impressed with the size and specifications of the device.”
The E-Series has been gaining admiration in the cardiac community because of the petite size of the devices, their 14-year expected battery life, and the award-winning MRI AutoDetect sensor. This function detects when the device is in an MRI environment and automatically switches it to ProMRI® mode. This limits the length of time a device remains in the limited functional state of ProMRI mode to the duration of the MRI scan only. It recently won the Cardiostim Innovation Award for “Best Practice Improvement.”
“The small size of Enitra allows for easy handling by physicians, leaves a very good cosmetic result for the patient. Their day-to-day comfort will be noticeably better, too,” stated Charlotte Pompei at BIOTRONIK UK. “Enitra also features BIOTRONIK Home Monitoring® and Closed Loop Stimulation. With its very long battery life of 14 years, it is a very appealing device for both patients and doctors.”
About ProMRI
BIOTRONIK ProMRI technology enables patients with a pacemaker, implantable defibrillator, cardiac monitor, or cardiac resynchronization therapy defibrillator (CRT-D) or pacemaker (CRT-P) to undergo an MRI scan. Devices with MRI AutoDetect have the additional capability of automatically recognizing an MRI environment within a programmable time window, switching on the device’s MRI mode for only as long as is required to complete the scan. This typically lasts around 30 minutes. This ensures the patient receives the full benefit of their device for the maximum amount of time possible. BIOTRONIK has the broadest portfolio of cardiac devices approved for use in the MR environment on the market. For more details, please go to www.biotronik.com/promri/en.