After only four months from contract sign-off, BSI CTMS will be rolled out at OCT at the end of June 2019. “We are happy that our clinical trial experts will be able to benefit from this state-of-the-art solution so soon. This is because the BSI CTMS covers all features that are relevant to us with its standard version,” explains Irina Petrova, Clinical Operations Director at OCT.
OCT is a leading full-service CRO that operates on behalf of well-known pharmaceutical and biotech companies. With a team of over 200 experts, OCT offers its customers the entire bandwidth of clinical trials and services that comprise project management, monitoring, data management, and statistics.
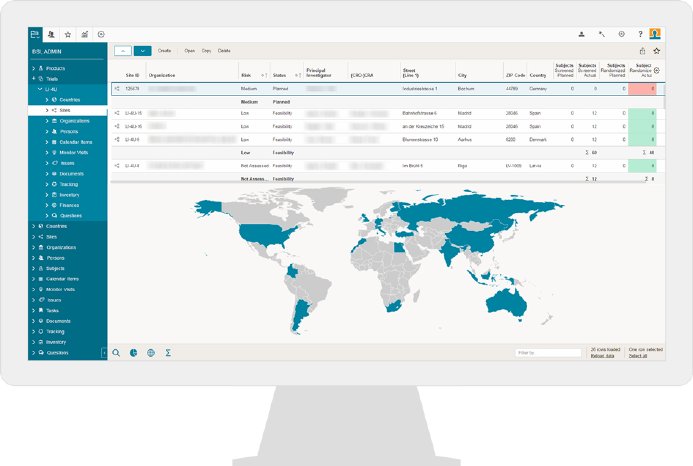
“The OCT team has performed a very thorough examination of a number of key CTMS vendors (10 in total). We have been evaluating our options based on such parameters as full-range of functionality, flexible design, availability of validation documents. In addition, OCT team was given an opportunity to receive feedback on the platform from several CROs, among which there were partner CROs, with whom OCT has been successfully cooperating for a long time. The BSI solution won us over. It covers all the aspects of clinical trial management, including electronic Trial Master File (eTMF), that allows a trial to be monitored online by sponsors, giving the assigned individuals access to the necessary trial documentation,” explains OCT’s choice Irina Petrova.
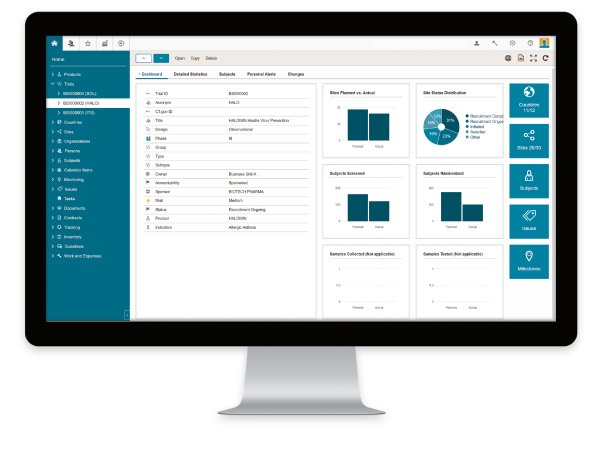
By using the eTMF document management module for specific trials, OCT, in particular, anticipates efficiency increases and an enhanced overview once the BSI CTMS has been launched. This module offers a clear presentation of all documents to be compiled and tracked. Also, OCT will be able to schedule and manage all project and monitoring activities, and with the integrated “Human Resource Management System,” the users can book all project efforts directly in the CTMS.
“We very much look forward to our partnership with BSI. It will allow us to optimize our processes in the clinical trial management area substantially”, says Dmitry Sharov, CEO of OCT. Sigrid Kristiansen, a Senior Business Specialist at BSI, adds: “It is great to see how BSI CTMS can map OCT's rather challenging requirements with standard features. It made our joint specification workshops with OCT almost effortless.”
About OCT
OCT is the leading clinical research organization (CRO) in Russia and a full-service CRO for clinical trials. The company has been conducting clinical trials successfully in central Europe, Ukraine, Georgia, Bulgaria, Latvia, Estonia, Lithuania, Belarus, Moldavia, Serbia, and the United States. Its more than 200 experts conduct clinical research for global sponsors including well-known pharmaceutical and biotech companies.
Links: www.bsi-software.com/ctms www.oct-clinicaltrials.com