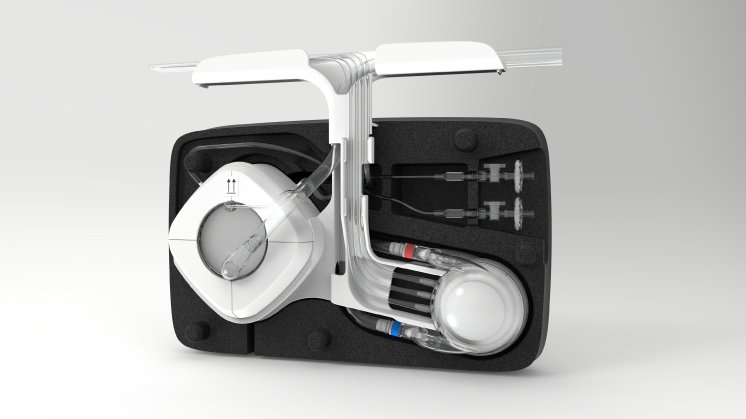
“I am repeatedly impressed at everything our team manages to achieve in such a short time. The 28-day authorization of the MOBYO® gas exchanger in particular will provide safer patient treatment for our users,” said Dr. Jürgen O. Böhm, CEO & CMO of Hemovent GmbH.
The MOBYO® gas exchanger is part of the MOBYBOX® ECLS system and is also naturally available separately. The innovative design of the MOBYO® ensures extremely low pressure reduction while providing highly efficient gas exchange; the creatively designed cylindrical membrane stacks prevent stagnation areas. This permits gentle blood flow behavior, resulting in greater safety and reliability in patient treatment. The MDR certification for the MOBYO® and the 28-day authorization are further significant steps for the company as it internationalizes its ECLS products.