The medical device company is headquartered in Kitzbühel, Austria, and has offices in Austria, Germany, and Portugal. "We want to empower interventional radiologists and oncologists with a full-fledged medical robotics platform that helps them achieve better patient outcomes through micro-invasive interventions." explains INS Marketing Manager Margarida Gonçalves.
About the motorized positioning arm
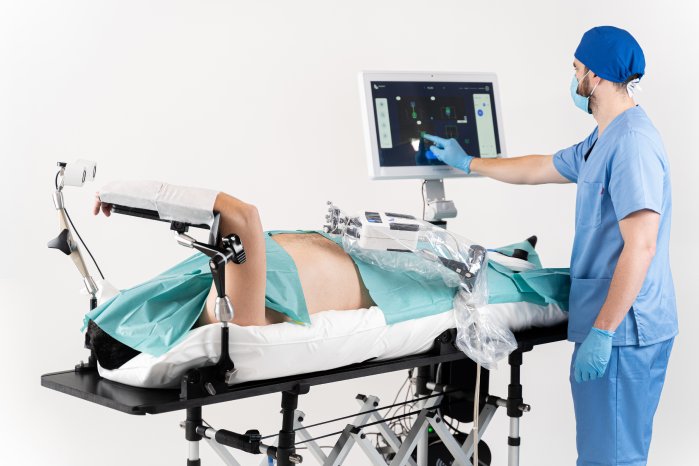
The motorized positioning arm is part of Micromate™, a head-to-toe medical robotics platform that helps doctors with an easy-to-use miniature robot for needle interventions. The motorized arm is intended for the positioning and holding of medical equipment for minimally invasive procedures.
Physically, it consists of a flexible mechanical arm with two spherical joints on each end and a simple middle “elbow” joint. It can attach to medical tables at one end and medical devices at the other. A weight of less than 2.5 kg ensures easy transportation, while still providing a steady holding force.
“With a self-locking mechanism, the motorized arm is an evolution of the purely mechanical positioning arm, where the locking and unlocking can be executed by pressing buttons. The operator can also easily swap instruments thanks to its universal quick-connector part.”, specifies Pedro Pinto, INS Systems Engineer. The arm also works under live imaging.
Challenge: a small and powerful battery was needed
“The positioning arm is powered by a battery, more exactly an 18650 1s1p lithium battery pack. With a capacity of 3350 mAh, we chose the solution with the most possible energy capacity for the smallest possible construction space.”, explains Jérémie Deloof, Sales Manager at Jauch. The battery had to be small enough to fit in the limited space and provide the maximum current drain required for the intended locking torque and locking speed of the arm. Of course, the battery also needs to be safe and durable for many locking/unlocking cycles between the charges.
Another very important topic is the passing of all tests to become medically approved. “The battery for INS has the UN38.3 and the IEC62133 certification. At Jauch we are able to test for the two certifications at our headquarter in Villingen-Schwenningen. Further, we are familiar with the regulatory requirements of the medical sector. Hence, we developed the PCM from the very beginning so that it would pass the demanding tests”, reports Deloof.
Further, especially in the MedTech industry it is essential to maintain the supply of battery cells. With the cell shortage being an issue at the start of the project, Jauch put a special focus on the search for alternative cells. Nevertheless, the company was able to choose and certificate an alternative cell to always be able to provide batteries to INS.
Why Jauch?
“The collaboration with Jauch was excellent. The team was always available and quick to reply to our questions, from further requested info on the battery’s performance to clearing doubts about the testing of the battery to the relevant standards. For us it was very important to collaborate with a company that can guarantee the quality of their products, which could undergo medical certification processes.” Pedro Pinto
Of course, the final question is: What is the current status of the product?
INS told us, that the motorized positioning arm has successfully passed all safety tests by ISO60601. The company is preparing to launch the product this year.