“The FDA approval of mediCAD® Web in addition to its already approved client-server version is another important step to create awareness of the necessity of digital preoperative planning in the US market and the world”, said Jörn Seel, CEO of mediCAD Hectec GmbH, Germany. “The interest in this new version of our software has already been very high in other markets around the world. This expands the capabilities of preoperative, digitized planning for surgeons in the orthopedic field. With this approval by the FDA, we now are pleased to bring this exciting new product development to the U.S. market and look forward to continuing to work with orthopedic surgeons to solve their planning needs.”
The mediCAD® portfolio of products are software tools that allow orthopedic surgeons to efficiently and safely plan joint, trauma and spine operations in a fraction of the time required for conventional planning. Further, with the automatic archiving of critical information and complete traceability of findings, mediCAD® provides a framework for monitoring and documenting surgical procedures.
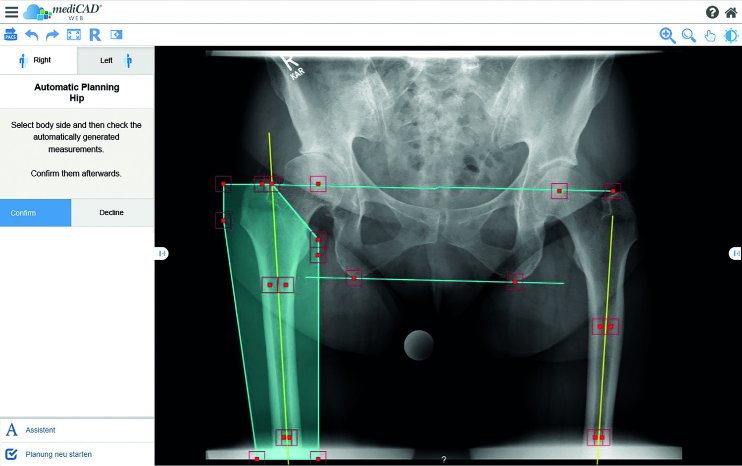
With its newest product mediCAD® Web approved for use in the U.S., the company has now brought a planning software into the market that brings even more flexibility in the planning process. mediCAD® Web is cloud-based and can easily be accessed from your tablet or any other device via the browser without installing any software. It is the new platform-independent planning tool of the classic version of mediCAD®.
With the mediCAD® Web solution and the approval of these products for use in the U.S., the company continues to set innovative milestones worldwide in supporting surgical orthopedics and maintains its position as a global leader in the field of medical software solutions.