The new Cloud Alpha 96 series was developed as a result of numerous customer requests and is now capable to expose mammalian cell cultures in Corning ® HTS Transwell ® 96-Well Permeable Support Systems. The Cloud system is suitable for nebulization of solutions and suspensions. Possible fields of application are screening of inhaled drugs, toxicity testing of inhaled substances such as chemicals or nanoparticles and virus research.
Exposure at the Air / Liquid Interface
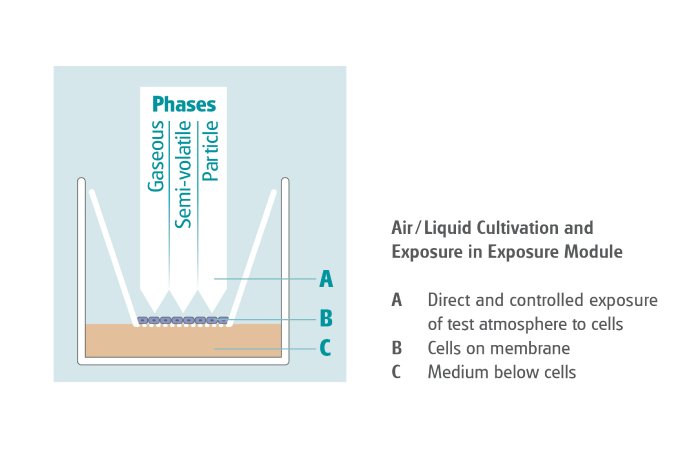
In response to the scientific need to expose in physiologically relevant conditions, the VITROCELL® Cloud Alpha exposure devices have been specifically designed to enable direct exposure of mammalian cells or tissue at the Air/Liquid Interface. Here the cell cultures are not covered with media as opposed to submerged conditions which cause an undesired interaction of the formerly airborne substances with the culture media.
Cell systems cultivated on membrane inserts are exposed at the Air/Liquid Interface (ALI) so that the test substances are received directly. This approach allows for more credible and authentic results than by submerged exposure due to a closer replication of the human physiology.
Two Versions available
The system is available as Cloud Alpha 96 and Cloud Alpha 96 +. The latter features an integrated Quartz Crystal Microbalance for online assessment of the deposited mass.
Dosimetry using Quartz Crystal Microbalance (QCM)
The QCM sensor is integrated in the Cloud Alpha 96 + exposure module. It is capable of measuring the deposited mass at a resolution of 10 nanogram/cm2 per second. Results are reported online by the VITROCELL® Monitor software. Data is presented in graphs and stored in MS Excel ®.
Choice of three types of nebulizers
The system comes with a choice of 3 types of vibrating mesh nebulizers having droplet MMAD ranges of 2.5 – 6.0 μm, 2.5 – 4.0 μm and 4.0 – 6.0 μm.
Recommended nebulisation volumes are 200 μl (Cloud Alpha 96) and 300 μl (Cloud Alpha 96 +). So the device is particularly suitable for testing whenever small quantitities of testing materials are available.
Key Features:
- Integrated controller for aerosol generator
- Optional integrated microbalance controller
- Defined experiment recipes
- Automatisation of the experiment by nebulization time or by user-defined volume
- Output rate database for nebulizers
- Heating system
- Optional PowerVent function: evacuation of potentially residual aerosols via vacuum pump
- Designed for screening of inhaled drugs, toxicity testing of inhaled substances such as chemicals or nanoparticles and virus research